
Metal Corrosion - An Introduction
Metal Corrosion, a Natural Electrochemical Process, poses a significant challenge in various industries, from infrastructure and transportation to manufacturing and electronics. In a world dominated by metal in everyday objects like cars and household appliances to critical infrastructure like bridges and pipelines, metal corrosion poses a significant threat. This pervasive phenomenon has the potential to compromise structural integrity, impair functionality, and incur substantial economic losses.
In this blog, we delve into the intricacies of metal corrosion, exploring its causes, effects, preventive measures, and mitigation strategies
Understanding Metal Corrosion.
Metal corrosion is a chemical reaction that occurs when metals come into contact with their surrounding environment, resulting in the deterioration of their properties. It is an electrochemical process driven by redox reactions. Most metals naturally want to return to their original oxidized state. This conversion is called corrosion. In the simplest sense, corrosion is the decay of metal molecules into its oxides. The results is degradation of chemical and physical properties of metal. The severity and degree of corrosion that happens over time are based on material and the operating environment.
Some metals don’t corrode deep, because when they oxidize, the oxides itself creates a thermodynamically stable seal around the rest of the metal. This passive film prevents further corrosion of layers farther down, in a process called passivation.
Copper’s patina, identifiable by its distinctive green color, is an example of a passive film that protects the metal underneath.
What is Rust?
Corrosion and rust are often used interchangeably. While corrosion and rust are both a result of oxidation of Metal, there are differences between the two.
Rusting, is a specialized form of corrosion that occurs only in iron and steel. In simpler terms, Iron Oxide, commonly known as Rust, occurs when steel or iron undergoes oxidation, resulting in the formation of iron oxide and causing it to gradually peel away.
Red iron oxides do not bond to the iron beneath them, but rather pull away and flake off. Therefore, rust does not provide a passivation layer, but instead corrodes away, exposing the next layer of iron, which then oxidizes, flakes, and constantly repeats. This can lead to structural failure of the Iron Metal over a period of time.
Causes of Metal Corrosion
Several factors contribute to the onset and progression of metal corrosion. Understanding these causes is vital for developing effective preventive measures. Here are some of the primary causes of metal corrosion:
- Moisture: The presence of moisture, especially in the form of humidity or liquid water, accelerates corrosion. Water acts as an electrolyte, facilitating the electrochemical reactions necessary for corrosion to occur.
- Oxygen: The presence of oxygen in the environment, especially in combination with moisture, is a significant catalyst for metal corrosion. Oxygen reacts with metal atoms to form metal oxides, process commonly known as oxidation, weakening the metal’s structure.
- Chemical Exposure: Metals can corrode when exposed to various corrosive substances or chemicals such as acids, alkalis, salts, and pollutants. These substances can react with the metal surface, by initiating or catalyzing the electrochemical processes, leading to corrosion.
- Temperature: Temperature and Heat: High temperatures can accelerate the corrosion process, increasing the rate at which metals deteriorate. Thermal cycling, where metal surfaces repeatedly undergo temperature changes, can also contribute to corrosion, as higher thermal energy facilitates chemical reactions.
Types of Metal Corrosion
Metal corrosion manifests in various forms, each with its unique characteristics and implications. Some common types of metal corrosion include:
- Uniform Corrosion: This type of corrosion occurs uniformly across the metal surface, resulting in a gradual thinning or loss of material. It is typically caused by exposure to moisture or chemicals.
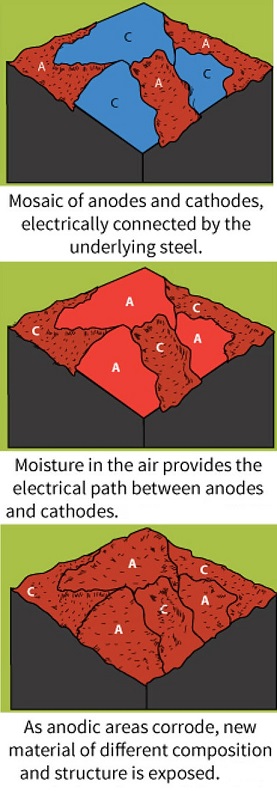
- Galvanic Corrosion: Galvanic corrosion occurs when two dissimilar metals come into contact in the presence of an electrolyte. The more reactive metal acts as an anode, corroding faster, while the less reactive metal acts as a cathode.
- Pitting Corrosion: Pitting corrosion is localized corrosion characterized by the formation of small pits or cavities on the metal surface. It can be initiated by the presence of impurities or localized damage on the metal.
- Crevice Corrosion: Crevice corrosion occurs in narrow gaps or crevices where stagnant electrolytes form, depriving the metal surface of oxygen and leading to localized corrosion.
Effects of Metal Corrosion:
Metal corrosion can have significant and diverse impacts, affecting functionality, safety, business interests, and even human lives. The common effects of metal corrosion encompass a wide range of consequences that can be critical in various contexts. While mentioned briefly, the effects of metal corrosion can be extensive and powerful, spanning a range of outcomes. These effects are not limited to, but can include:
- Structural Damage: Corrosion weakens the structural integrity of metals, leading to deformations, cracks, and eventually, failure. This poses a severe risk in applications such as bridges, buildings, and pipelines.
- Reduced Efficiency: In industrial processes, corrosion can interfere with the smooth operation of machinery, reducing efficiency and productivity. It can also impact energy transfer, leading to increased energy consumption.
- Financial Loss: Corrosion-related maintenance, repairs, and replacements can incur significant costs for industries and individuals. Additionally, the economic impact of downtime and production losses adds to the financial burden.
Prevention and Mitigation
Preventing and mitigating metal corrosion is crucial to ensure the longevity and reliability of metal structures and components. Consider the following preventive measures:
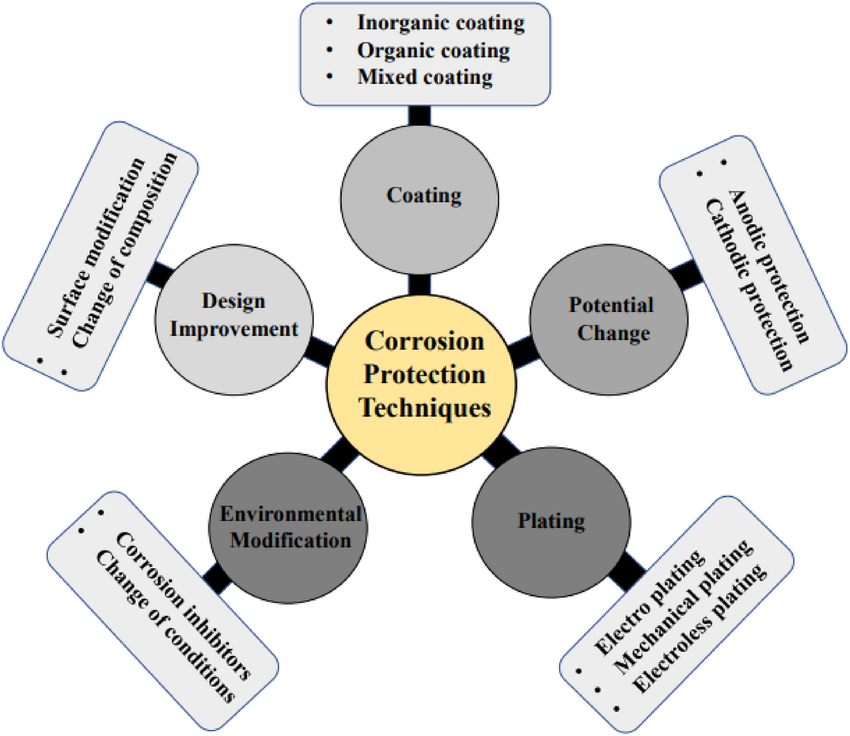
- Protective Coatings: Applying protective coatings like paints, varnishes, or metal plating can create a barrier between the metal surface and the corrosive environment.
- Cathodic Protection: Cathodic protection involves the use of sacrificial anodes or impressed current systems to shift the metal’s potential and prevent corrosion.
- Alloy Selection: Choosing corrosion-resistant alloys appropriate for the specific application can significantly reduce the susceptibility to corrosion.
- Environmental Control: Regulating temperature, humidity, and chemical exposure can help mitigate corrosion by minimizing the corrosive factors.
- Regular Maintenance: Implementing routine inspections, cleaning, and maintenance procedures can identify early signs of corrosion and allow for timely intervention.
Protective Coating – An Insight
Fortunately, there are several Natural, Artificial and Non-Metallic solutions that can help slow down or even completely stop corrosion from occurring on metals. Many iron and steel products are protected by being coated in a hard layer of another substance. These include:
- Natural Coatings: Naturally occurring substances such as beeswax, vegetable oil, lanolin, animal fat, and animal leather, offer a viable option for protecting metal surfaces against oxidation and corrosion. These naturally occurring substances can be utilized to slow down or prevent the occurrence of corrosive reactions on metals. Each product possesses unique properties and capabilities, making it important to select the most suitable option based on the specific use case requirements.
- Polymerized oil: Linseed oil (made of flax, but not to food-grade standards) was one of the original finishes to wrought iron products. A smith might quench in oil or paint an object with oil and then torch it, providing the traditional black-brown luster that we still emulate on wrought iron today—usually with lacquers, paints, or varnishes.
- Hot-dip galvanization: In this process, zinc is applied to steel or iron in a molten bath. Zinc galvanization has several protective properties. Firstly, it creates a patina, which seals both the zinc and the iron or steel below. Secondly, it is more “active” than the metals it is applied to. Over the life of a galvanized steel object, the zinc acts as the “sacrificial anode.” As it degrades, it protects and slows the corrosion of the steel underneath, even should that steel be exposed.
- Electroplating: Electroplating is a process of depositing a thin layer of metal onto the surface of an object through an electrochemical reaction. It involves immersing both the object to be plated (known as the substrate) and a metal electrode into an electrolyte solution. By applying an electric current, metal ions from the electrode are attracted to the substrate, where they form a metallic coating. This causes electrical bonding that is uniform and unbroken over the surface of the metal.
- Powder-coat: Powder coating is a dry finishing process in which a fine powder composed of resin, pigment, and additives is electrostatically applied onto a surface of the object. The powder is then cured through heat, forming a tough, protective, and visually appealing coating. The coat will maintain luster for years, providing superior protection and requiring minimal upkeep.
- Paint: Both indoors and out, and even in the heart of high performance engines, paint is a popular and versatile sealant. As an inexpensive sealant it still does a very good job at protecting iron and steel from the elements. When it cracks and wears down, it is simple enough to sand and reapply. Paint’s drawback is its own tendency to crack and flake in weather: even a small hole in the sealant layer can start the process of rust below. However, these drawbacks are sometimes manageable over the use of a part or part of a regular maintenance routine.
- Non-Metallic Coating: Non-metallic materials, including plastics, ceramics and paper, can effectively mitigate the risk of corrosion due to their inherent properties. Unlike metals, these materials do not undergo the same chemical reactions with oxygen, which significantly reduces the likelihood of corrosion occurring.





Caring for Metal Objects
Metal is a classically durable material, used to create objects and structures to last over many lifetimes. Most metals are vulnerable to corrosion and electrochemical decay. When evaluating materials for a project, taking into account the environment in which the metal will be used, enables a designer to select metal that withstands those conditions most effectively.
Iron is very prone to oxidization, and rust will usually set in with the slightest hint of water and oxygen. Unlike other metals, this oxidization will not form its own sealing passive layer. Therefore, sealants are usually used with ferrous metals. Finding the right type and keeping an eye on the metal over the decades of its use can help prolong the life of any metal object.
